About the 4th Cell Therapy Potency Assay Summit
The 4th Cell Therapy Potency Assay Summit enhanced quality control content and a balanced focus across cell types and disease indications. This summit was the crucial forum for staying ahead of the latest FDA regulatory updates and gaining insights from industry leaders who have dealt directly with regulatory bodies. In 2026, make sure you are involved in the go-to event for analytical, quality control, and regulatory experts to share development strategies and the latest technologies that may streamline your assay development workflows.
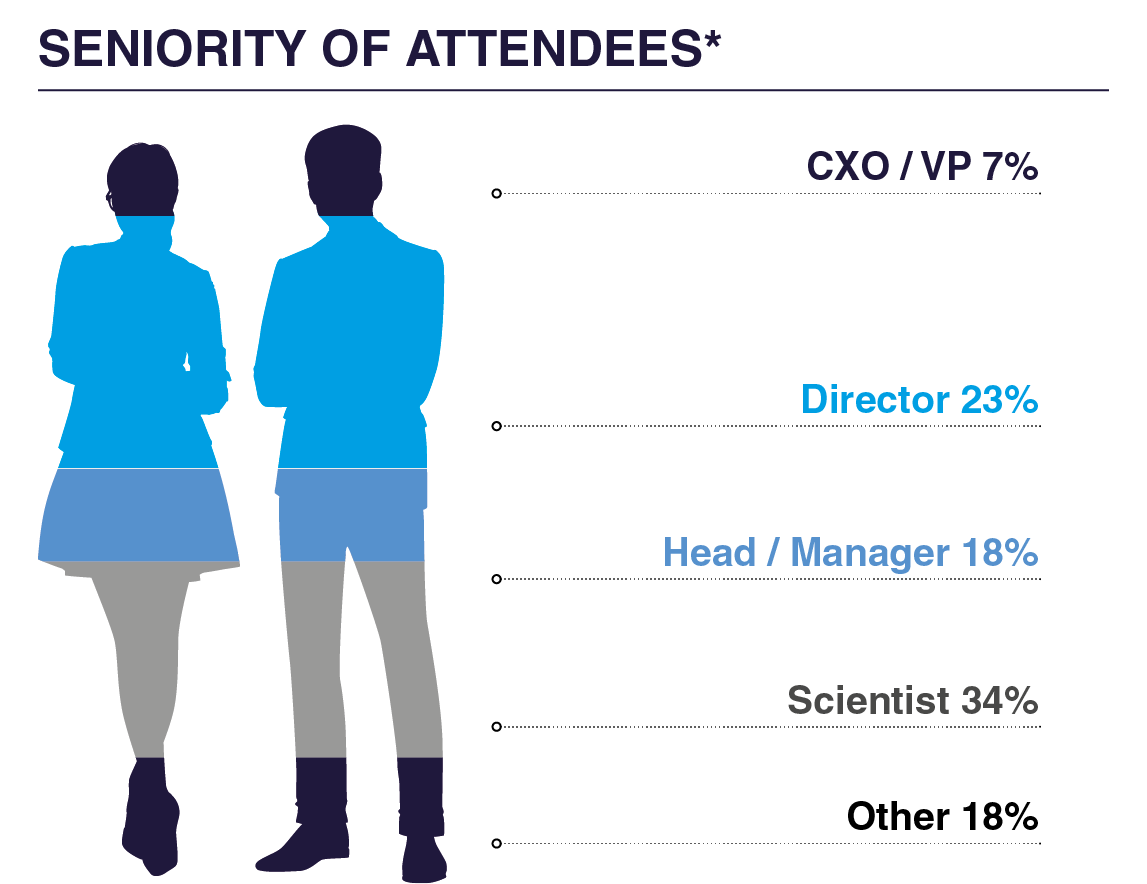
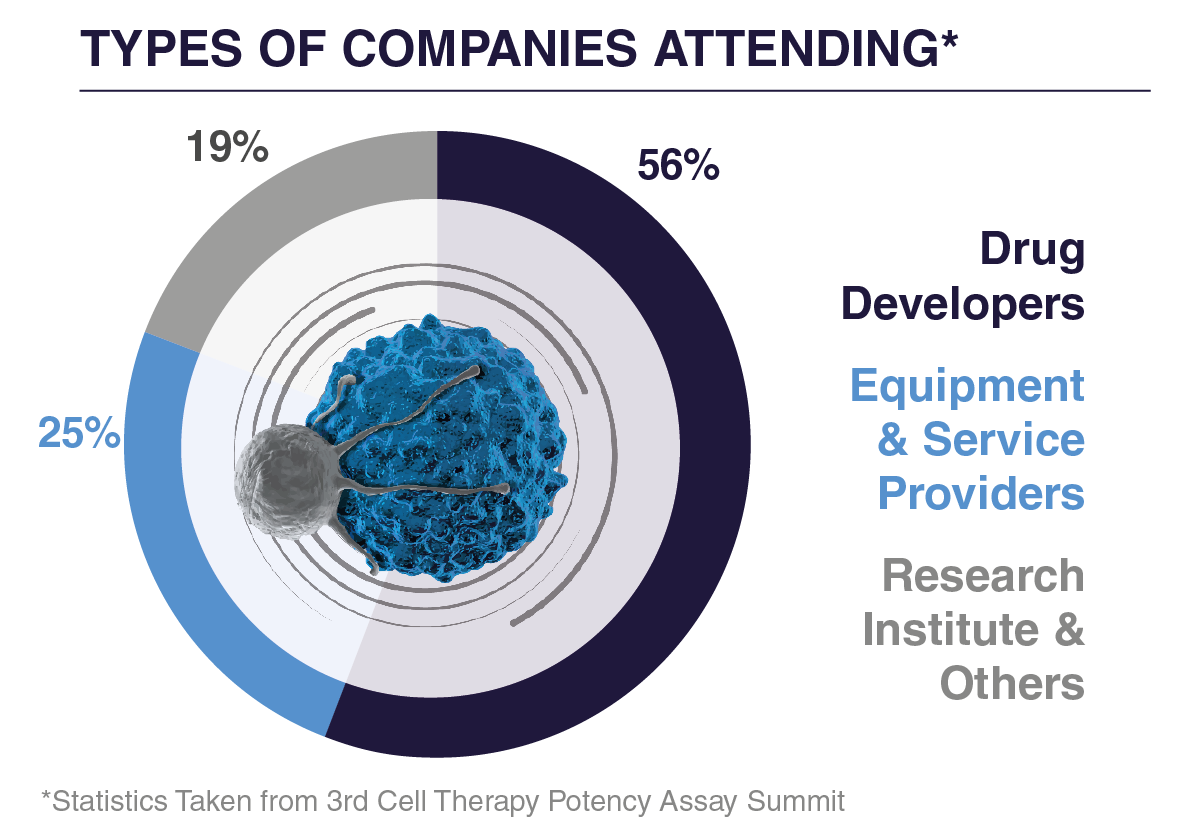
WHO ATTENDED IN 2025?

What You Missed:
Gaining insights from experts at BlueRock Therapeutics, Bristol Myers Squibb and Vertex Therapeutics on the latest advancements in potency assay development
Learning about the new FDA potency guidance and how to implement these requirements effectively in your development processes
Discovering cutting-edge technologies for assay quantification, including impedance assays and closed systems, to enhance accuracy
Deep diving into quality control strategies with increased content on ensuring product consistency and reducing batch variability
Who Could You Meet in 2026?
“I liked the opportunity to meet with others in the area and to share our experiences. It’s an excellent venue for learning the latest and for networking”
~ Head of Analytical & Translational Sciences, Tigen Pharma, 2024 Speaker
“The selection of speakers and panelists were very knowledgeable, passionate and approachable. The event was very organized, from the timing of each talk, the breaks and the planned opportunities for networking”
~ Head of Assay Development, Kiromic Biopharma, 2024 Speaker
